Hcl Ba Oh 2
Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides ROOH or in atmospheric chemistry by the reaction of excited atomic oxygen with water. Provided base is BaOH2 Provided acid is H2SO4 One molecule of BaOH2 dissociate to give two OH- Q.

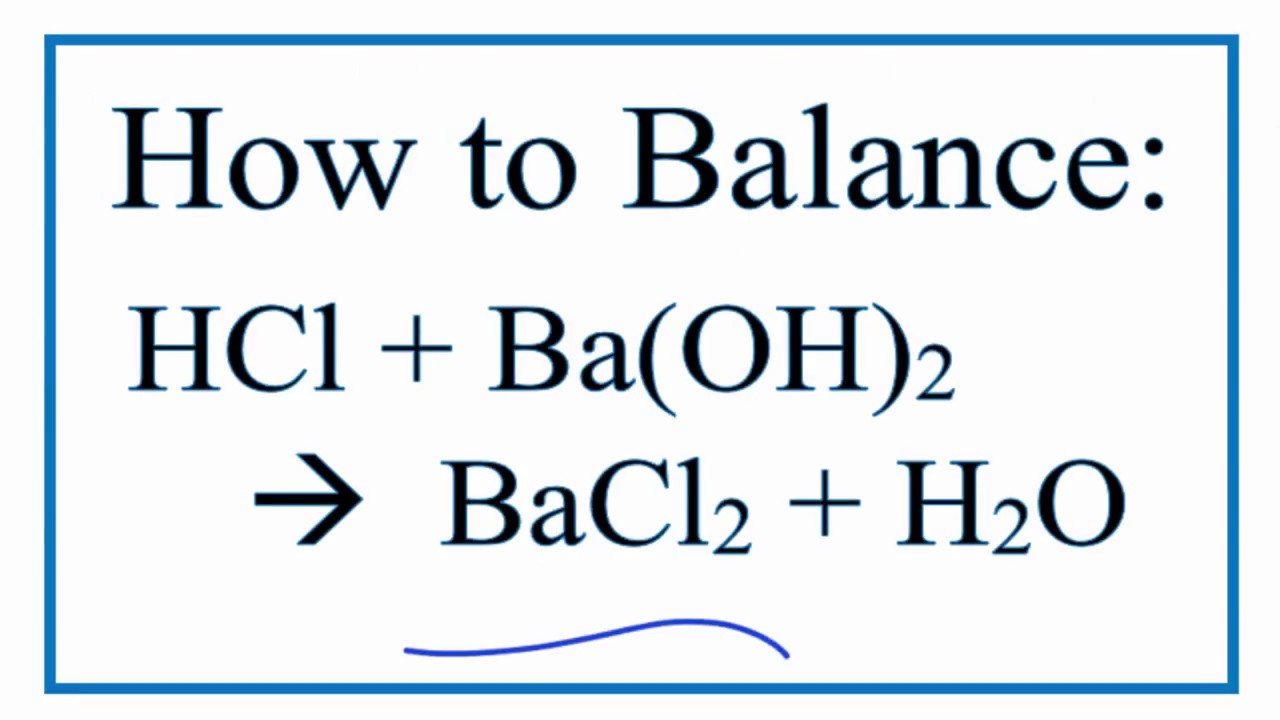
Type Of Reaction For Hcl Ba Oh 2 Bacl2 H2o Youtube
Caracterdesign Getty Images Great job.
. Oxalate effects a similar reaction. Соляную кислоту получают растворением газообразного хлороводорода HCl в воде. A How many milliliters of 0120 M HCl are needed to completelyneutralize 500 mL of 0101 M BaOH2 A.
In aqueous solution BaCl 2 behaves as a simple salt. Create an equation for each element H Cl Na O where each term represents the number of atoms of the element in each reactant or product. A double replacement reaction will occur if a formation of a precipitate gas or water takes place.
The coordination number of Ba 2 increases from 9 to 10. Solve For All Variables. 0a 1 b 1 c 0d O.
You did so well on this quiz you could tutor others on how to balance equations. B Calculate the number of moles of H2SO4. From the reaction it is clear that 2 moles of NaOH.
A KMnO 4 b HCl c KCl d MnCl 2 f Cl 2 g H 2 O Create a System of Equations Create an equation for each element K Mn O H Cl where each term represents the number of atoms of the element in each reactant or product. H 3 po 3 はhpooh 2 側に互変異性化するこの化学種の平衡はpoh 3 側が劣勢であるトリヒドロキシ型は亜リン酸ジヒドロキシ型はホスホン酸と呼ばれるリン酸のいくつかはoとpとの間でのhの移動により複雑に互変異性化する. Create a System of Equations.
For each of the water soulble compounds indicate the ions present in and aqueous soultion. Select two compounds above and this calculator will predict whether or not the reaction will occur in waterThis is simply based on the solubility chart of inorganic compounds. It is pervasive in some situations.
Click hereto get an answer to your question What is the OH - in the final solution prepared by mixing 200 mL of 0050 M HCl with 300 mL of 010 M BaOH2. Το υδροχλωρικό οξύ είναι υδατικό διάλυμα του αέριου υδροχλωρίου με το οποίο έχει και τον ίδιο χημικό τύπο HClΕίναι ανόργανο ισχυρό οξύ πολύ διαβρωτικό με πολλές και σημαντικές βιομηχανικές χρήσεις. 氢氧化钯iv pdoh 4 524710 14.
Standard Cathode Reduction Half-Reaction Standard Reduction Potential E volts Li aq e-rightleftharpoons Lis-3040. When this happens its usually because the owner only shared it with a small group of people changed who can see it or its been deleted. 1 a 0b 1 c 0d Na.
Ba 2 SO 2 4 BaSO 4. 物质 化学式 0 c 10 c 20 c 30 c 40 c 50 c 60 c 70 c 80 c 90 c 100 c 氢氧化钯ii pdoh 2 410610 10. The hydroxyl radical is the diatomic molecule OHThe hydroxyl radical is very stable as a dilute gas but it decays very rapidly in the condensed phase.
Хлороводород получают сжиганием водорода в хлоре. 1 a 1 b 0c 2 d Cl. Its solutions react with sulfate ion to produce a thick white precipitate of barium sulfate.
A HCl b NaOH c NaCl d H 2 O. Оксид бериллия является одним из 2 так же существует оксид бериллия 1 бинарным соединением бериллия с кислородом хотя в паровой фазе над ВеО при температуре около 2000 С было отмечено. In water it is a 12 electrolyte and the solution exhibits a neutral pH.
Rb e-rightleftharpoons. 0a 1 b 0c 1 d. If youre feeling a bit shaky on all the steps and details you can review the simple method of balancing equationsOtherwise you may wish to review how to balance oxidation-reduction or redox reactions or move on to understanding mole relations.

How To Write The Net Ionic Equation For Hcl Ba Oh 2 Bacl2 H2o Youtube

How To Balance Hcl Ba Oh 2 Bacl2 H2o Hydrochloric Acid Plus Barium Hydroxide Youtube


Comments
Post a Comment